
Industry summit
Enhancing
pharma and nutrition
processes
Hosted at DIOSNA in Osnabrück, Germany
24-25 June 2025
An exclusive
industry summit
hosted by Coperion
We are happy to welcome you at our Coperion Industry Summit: Enhancing Pharma and Nutrition Processes on June 24-25, 2025, at DIOSNA in Osnabrück, Germany – a brand of Coperion. This event is specifically designed for developers and manufacturers of pharmaceutical, nutraceutical, personal care products, and dietary supplements. Join us to enhance your production and development requirements and increase overall yields, and improve overall efficiency in batch & continuous manufacturing.
Gain valuable insights and improve your professional expertise through informative lectures led by respected industry experts. Secure Your Spot! Attendance is limited to 100 participants. The registration fee is €65. Register now to become part of an exclusive network of industry professionals. We look forward to you joining us and sharing meaninful learnings together!
With the participation of technology experts from




What to expect?
Expert Talks: Our speakers will provide important insights on key topics impacting your industry also while addressing current trends and challenges in production. These informative presentations will enable you to:
- Learn about insights into modern contract manufacturing and facility development in Germany from Dr. Johannes Trapp Losan (CDMO), with a focus on effective pellet production processes.
- Deepen your understanding of containment including the latest developments on flexible isolation through a presentation by expert Mike Brown from ONFAB.
- Engage in discussions with James Scicolone from the Department of Chemical and Biochemical Engineering from Rutgers University, NJ, on the groundbreaking studies done without the use of blenders in continuous direct compression manufacturing lines.
Understand the advantages of hot melt extrusion for pharmaceutical products and how modeling of the complete unit can help shorten and improve equipment configuration , as illustrated by Josip Matic of RCPE, Austria.
Learn the true requirements and definitions in ATEX classifications, specifically for equipment and environment in pharmaceutical operations.
Observe live demonstratons of a medicinal gummy line and understand the complete process for this growing type of nutraceutical and pharmaceutical dosage form.


Key Processes of Focus
- Automation of ingredients and system
- Mixing, granulating, drying, milling, sieving, spheronizing, and extruding
- Forming, coating, conveying, and material transfer
- Homogenizing and emulsifying
- Liquid depositing and filling
All presentations will be technical in scope and well suited for engineers, operators and plant managers who are looking to integrate and understand a variety of the latest technologies into their processes. It will educate the attendee on how to improve overall product quality and increase process yields by choosing the most accurate and innovative equipment designs available.
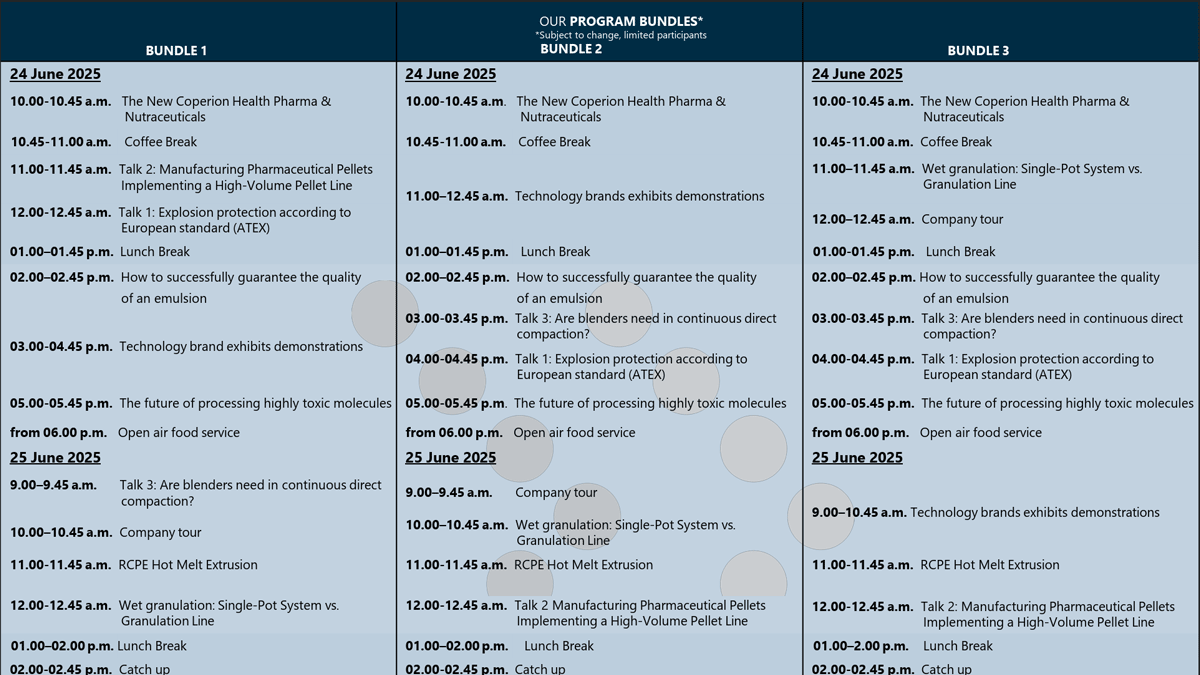
Event program
Subject to availability – Limited number of participants
You can choose from three bundles. The program content is the same. Only the program items are arranged differently in order to hold the lectures in small groups as far as possible. Please note that the number of group participants per group is limited. Depending on availability, we may allocate you to the next available group. Please note that we have tried to organise as many events as possible in small groups. Nevertheless, some presentations will be held in the whole group. Program subject to change.
Live demonstrations and equipment showcases

Servoform
Mini Depositor
- Production of vitamin gummies or medicated hard candies
- Small batch production up to 440pcs/min
- Accurate control of API content
- Capable of GMI validation

CM170
Lab Unit Mill
- Exhibit with 170mm core diameter
- High efficiency – virtually all the energy input is utilized in the size reduction process
- Permits uniform size distribution, resulting in the minimum of fines
- Low heat generation – essential when milling fatty, sticky or heat sensitive products
- Low dust levels – no need for air

Extruder DE-40
with spheronizer R250
Extruder DE50, length 10D
- Wet extrusion for moistened powder masses (eg. MCC, lactose)
- Co-rotating and intermeshing screws
- Throughput rates up to 40kg/h (depending on product)
- Exchangeable dies
- Champshell design, easy-to-clean
Spheronizer R250
- GMP design for pharma industry
- Disc radius 250mm
- Up to 0.5kg/batch
- Spheronization time 1-5mn
- Size of pellets 0.4-5mm
- Exchangeable discs with various patterns

P 1-6 Laboratory
Mixer Granulator
- Short mixing times
- Homogeneous mixtures
- Plug & play handling
- Toll-free scale-up
- Optional with spheronizer for pellet production

QT20 Feeder
with P10 receiver
- Receiving of excipients and active ingredients in mills and micronisers
- Pharmaceutical receivers for melt and wet granulation extrusion
- Dispensing and receiving of one or more active ingredients, including reactor feeding
- Lubrication of tablet compression tools
- Receiving in continuous pharmaceutical processes such as mixing/blending, wet and dry granulation, coating and drying

Trilab 10
vacuum homogenizer
- Thermally controlled vacuum homogenizer with triple agitation
- Versatility of applications from 10 to 50 litres
- Scale-up for formulations for industrial manufacturing
- Recipe control and repeatability
Register and save your seat today
Registration fee €65 plus VAT, no refund.
Limited seats available. Visit the DIOSNA webpage to save your seat and check accommodation information. See you soon in Osnabrück!
Register today